Mississauga is the sixth largest city in Canada. It is in the south of the province of Ontario in the region of peel. The city is estimated to have a population of around 715,000 people. The rapid growth of this city is attributed to its proximity to Toronto. It was originally built as suburb of Toronto. Education in Canada is mostly free and publicly funded. It is overseen by the federal, provincial and local governments, with the education within provincial jurisdiction and the curriculum overseen by the province. Education is compulsory in most provinces up to the age of 16. Mississauga is served by the Peel District School Board, which operates the secular English speaking public schools. There is also a school board that runs the local catholic schools and a board that runs the French speaking schools in the area, one for the secular French schools and one for the catholic French schools. There are a number of schools in the area that offer specialised programs. These include the French immersion schools such as Clarkson Secondary School and Streetsville Secondary School. There are three schools in the areas that off a Regional Arts Program and two that offer a specialist sci-tech program for students who are gifted in these areas. There is also two schools that offer support for those studying for the international baccalaureate plus numerous other programs. Canada’s higher has a very good reputation. However there is no formal ranking system and students will often choose colleges and universities bases on geographic convenience and the reputation of a particular course. Mississauga is home to one of the three campuses run by the University of Toronto. This university is ranked as the 34th best university in the world and is the second highest ranked Canadian university in the rankings. The campus was established in 1967 and is the universities second largest division. It is home to 12,000 students. They have 15 academic departments and pupils can choose from 148 programs to participate in from 89 areas of study. Students can study both undergraduate and graduate programs on this campus. This campus also has an excellent research environment that has helped the staff and researchers become internationally recognised for their work. The university includes Institutes for Management and Innovation, and Communication, Culture, Information and Technology.
Free Wi-Fi
To make sure you’re always connected we offer completely free and easy to access wi-fi.








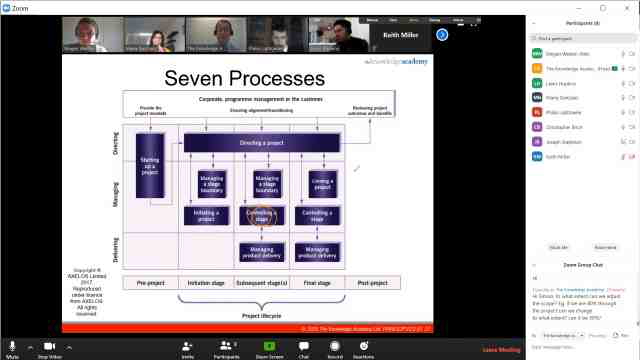
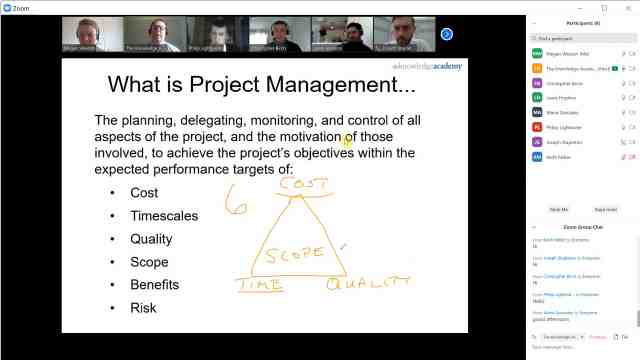


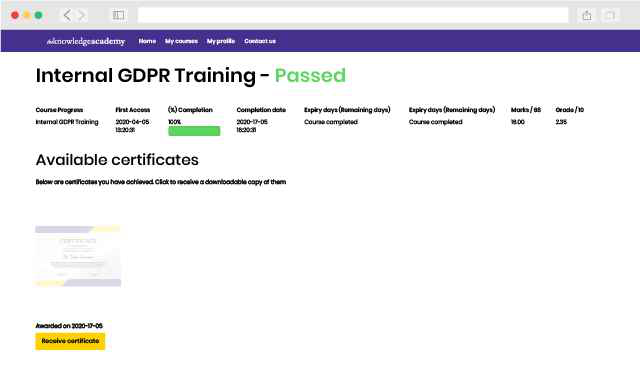
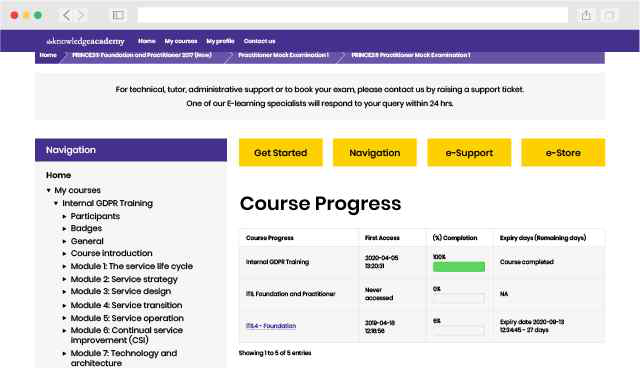















 Back to course information
Back to course information




 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please

