Get a custom course package
We may not have any package deals available including this course. If you enquire or give us a call on +1 7204454674 and speak to our training experts, we should be able to help you with your requirements.
Module 1: Introduction to ISO 13485:2016
Module 2: ISO 13485 Clauses 1 and 2
Module 3: ISO 13485 Clauses 3 and 4
Module 4: Requirements and Quality Systems
Module 5: Overview of ISO 13485:2016 Requirements
Module 6: Implementation Phases of the ISO 13485 Frameworks
Module 7: Conducting an ISO 13485 Certification Audit
Module 8: Relationship Between ISO 13485 and ISO 9001
Module 9: Internal Auditing
Module 10: Internal Audit Plan
Module 11: Audit Process
Module 12: Internal Audit Evidence and Findings
Module 13: Roles and Responsibilities
Module 14: Resource Management and Product Realization
Module 15: ISO 13485 and Quality Management Systems
Module 16: Principles of Quality Management System
Module 17: Fundamentals of Quality Management Systems
Module 18: Measurement, Analysis, and Improvement
Module 19: Risk Management


The ISO 13485 Lead Implementer Course in the United States gives employees the knowledge and skills they need to lead the implementation and management of an ISO 13485-based Quality Management System for medical devices. Below are some professionals who can benefit from this course:
There are no formal prerequisites for this ISO 13485 Lead Auditor Course. However, prior experience in medical device manufacturing or regulatory compliance will greatly benefit the delegates
ISO 13485 Lead Implementer Training in the United States is a course that introduces delegates to the aspects of managing quality in the medical device industry. ISO 13485 Certification is a globally recognized standard that establishes quality management system requirements for organizations that produce medical devices. Understanding its relevance is crucial, as it ensures that organizations consistently meet customer and regulatory requirements in the medical device field.
Mastering ISO 13485 is essential for anyone responsible for implementing and managing quality management systems in the medical device industry in the United States. It provides a valuable credential for individuals dedicated to quality assurance and regulatory compliance, opening opportunities for high-profile jobs and enhanced earnings. Its importance lies in its ability to enhance an organization's overall performance and mitigate risks associated with medical devices.
The 3-day ISO 13485 Training Course in the United States equips delegates with the necessary knowledge and skills to implement a quality management system for medical devices effectively. Participants will gain an in-depth understanding of the auditing process, enabling them to obtain audit evidence and evaluate it objectively. They will also learn how to control nonconforming products identified during the inspection process.
Course Objectives:
At the end of this training in the United States, delegates will be able to implement this ISO standard by specifying company requirements and the scope of medical devices. They will also be able to analyze the data and implement identified opportunities for improvement.




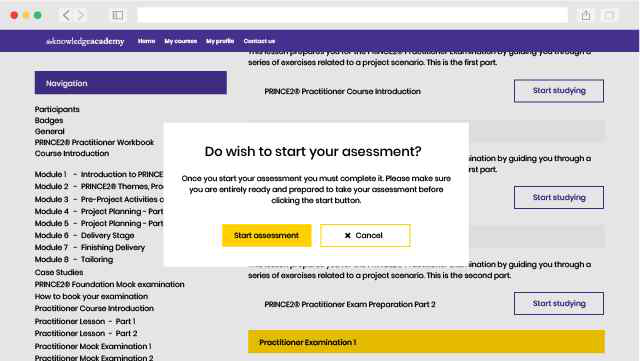
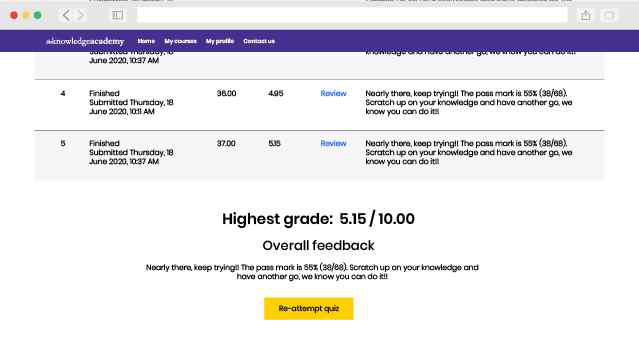
ISO 13485 Lead Implementer Exam Information
To achieve the ISO 13485 Lead Implementer, candidates will need to sit for an examination. The exam format is as follows:


Why choose us
Experience live, interactive learning from home with The Knowledge Academy's Online Instructor-led ISO 13485 Lead Implementer. Engage directly with expert instructors, mirroring the classroom schedule for a comprehensive learning journey. Enjoy the convenience of virtual learning without compromising on the quality of interaction.
Unlock your potential with The Knowledge Academy's ISO 13485 Lead Implementer, accessible anytime, anywhere on any device. Enjoy 90 days of online course access, extendable upon request, and benefit from the support of our expert trainers. Elevate your skills at your own pace with our Online Self-paced sessions.
The trainer was excellent. She was very knowledgeable, made the course content interesting (as it could have come across as death by PowerPoint) and very approachable,
I would like to provide positive feedback regarding the Lead Audit Certification ISO training, specifically highlighting the exceptional skills and expertise of the trainer, John. The training program was truly enlightening and informative. John's ability to convey complex concepts in a clear and concise manner was commendable. He demonstrated a deep understanding of ISO standards and effectively guided us through the entire auditing process. John's patience and willingness to answer all our questions made the training experience even more valuable. His interactive teaching style kept us engaged throughout the sessions, making it easier for us to grasp the practical application of the ISO standards. Furthermore, John's extensive industry experience was apparent in the real-life examples and case studies he shared, adding relevance and context to the topics discussed. His ability to relate to our specific industry challenges was highly appreciated.
Very interactive session
There hasn't been any questions asked about this Topic

You won't find better value in the marketplace. If you do find a lower price, we will beat it.

Flexible delivery methods are available depending on your learning style.
Resources are included for a comprehensive learning experience.




"Really good course and well organised. Trainer was great with a sense of humour - his experience allowed a free flowing course, structured to help you gain as much information & relevant experience whilst helping prepare you for the exam"
Joshua Davies, Thames Water



 Atlanta
Atlanta New York
New York Houston
Houston Dallas
Dallas Denver
Denver Seattle
Seattle Los Angeles
Los Angeles Chicago
Chicago San Francisco
San Francisco Philadelphia
Philadelphia San Diego
San Diego Phoenix
Phoenix Boston
Boston Austin
Austin Detroit
Detroit San Jose
San Jose Tampa
Tampa Colorado Springs
Colorado Springs Portland
Portland Sacramento
Sacramento Minneapolis
Minneapolis San Antonio
San Antonio Irvine
Irvine Las Vegas
Las Vegas Miami
Miami Bellevue
Bellevue Pittsburgh
Pittsburgh Baltimore
Baltimore Fairfax
Fairfax Orlando
Orlando Raleigh
Raleigh Salt Lake City
Salt Lake City Columbus
Columbus Oklahoma City
Oklahoma City Nashville
Nashville Charleston
Charleston Columbia
Columbia Cleveland
Cleveland Cincinnati
Cincinnati Memphis
Memphis Richmond
Richmond Virginia Beach
Virginia Beach Louisville
Louisville Fort Lauderdale
Fort Lauderdale Indianapolis
Indianapolis Des Moines
Des Moines Grand Rapids
Grand Rapids New Orleans
New Orleans Wichita
Wichita Charlotte
Charlotte Hartford
Hartford New Jersey
New Jersey Anchorage
Anchorage Omaha
Omaha Honolulu
Honolulu Albuquerque
Albuquerque Baton Rouge
Baton Rouge Iowa City
Iowa City Albany, NY
Albany, NY Boise
Boise Milwaukee
Milwaukee Tucson
Tucson Kansas City
Kansas City St Louis
St Louis Jacksonville
Jacksonville
 Back to course information
Back to course information
We may not have any package deals available including this course. If you enquire or give us a call on +1 7204454674 and speak to our training experts, we should be able to help you with your requirements.
 If you miss out, enquire to get yourself on the waiting list for the next day!
If you miss out, enquire to get yourself on the waiting list for the next day!

close


Press esc to close

close
Fill out your contact details below and our training experts will be in touch.



Back to Course Information