New Delhi is one of the districts within Delhi, as well as it being the capital city of India, it is also the home to the Government of the National Capital Territory of Delhi and some of the branches of the Government of India, these being the judiciary, executive and legislative branches and is also home to around 249,998 people. The capital of India - New Delhi was initiated within 1931 by Viceroy Lord Irwin however formed during 1911 by George V, who was the King of the United Kingdom as well as the Emperor of India and his British architects Sir Herbert Baker and Sir Edwin Lutyens formed the foundation stone and announced the capital city of India would be changed. The primary schools and secondary schools within New Delhi are either run privately or by the government and as of 2004 to 2005 there was around 1.5 million students going through the educational system within 2416 primary schools, 822,00 students in 715 middle schools and 669,000 students in 1576 secondary schools and around 500,000 students within 165 universities and colleges. All universities are either state, deemed or central universities, The top universities within New Delhi are the Jamia Millia Islamia, the University of Delhi, Indian Statistical Institute, National Law University, Jawaharlal Nehru University, Guru Gobind Singh Indraprastha University, Ambedkar University Delhi, Jamia Hamdard, Delhi Technological University – which is the first Engineering college within Delhi and one of the oldest in India, the Indira Gandhi National Open University is the largest national university in the world with over 4 million students, New Delhi is also home to the Indian Agricultural Research Institute, Pusa, New Delhi, Premier institute for agricultural research and education in India. The university courses and programs can offer different types of degrees such an undergraduate and post graduate courses, Bachelor courses and Scholarships, New Delhi prides itself on its premier institutions as the teaching and researching they do means that within 2011 the census recorded the literacy rate for females as 80.9% and for males it was slightly higher at 91.0% with an overall literacy rate of 86.3%. Subjects within the different universities can include, social sciences, engineering, humanities, technology, sports, law, social work, arts, management, education, computer information technology, foreign languages, mathematical science, dentistry and medical.
Free Wi-Fi
To make sure you’re always connected we offer completely free and easy to access wi-fi.










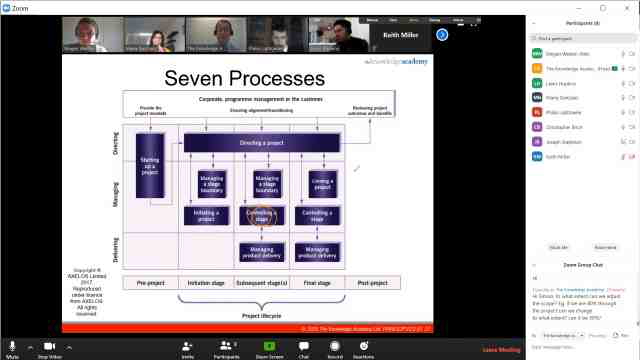
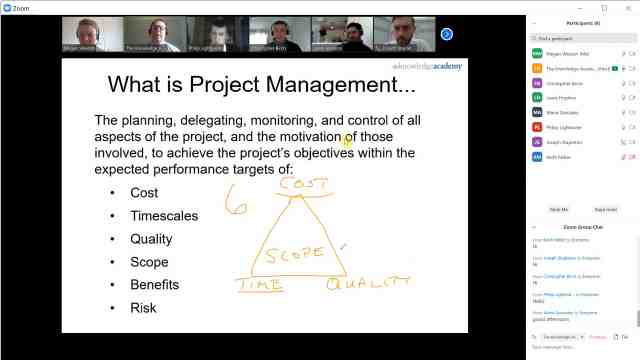





















 Back to course information
Back to course information




 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please

